Electroacupuncture attenuates chronic inflammatory pain and depression comorbidity by inhibiting hippocampal neuronal apoptosis via the PI3K/Akt signaling pathway
Kristen Sparrow • August 12, 2023

Click on photo or here for brief youtube explanation
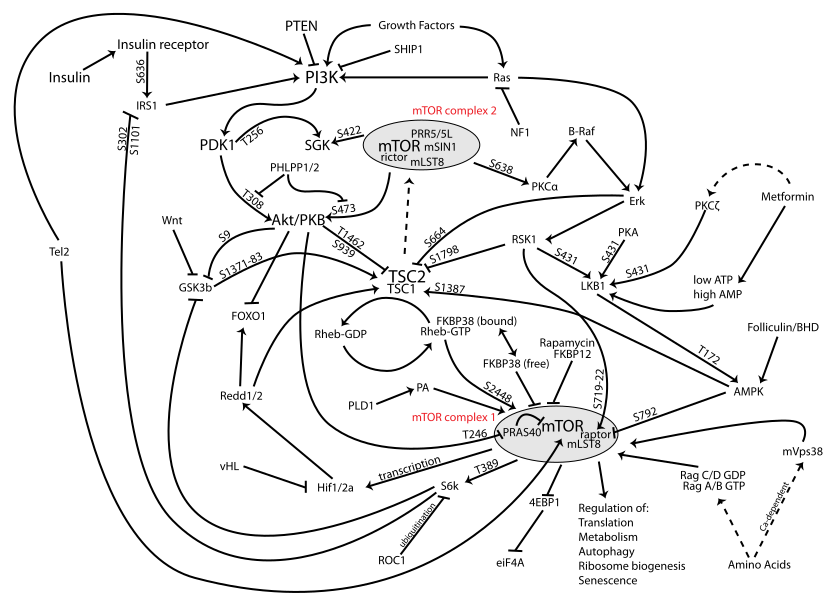
This study looks at mice in which chronic inflammatory pain has been induced experimentally. With this inflammation, changes in the hippocampus, the seat of memory. Instituting Electroacupuncture at LI4 and Li3 (a common point combination for pain) not only reversed the changes in the hippocampus but also decreased the clinical pain response and depressive response. It also led to an activation of the PI3K/Akt signaling pathway. This pathway is implicated in mTor, or the target of rapamycin, implicated in regulation of aging. We’ve discussed acupuncture and mTOR here and here.
(I included a diagram of the mTOR pathway below… yeesh!)
The problem of translating animal research to humans is frought with trouble. But also, to figure out how to be able to give daily acupuncture doses in humans clinically that could achieve these same results? This is no reason to discount these studies! But it’s always in the back of my mind.
Abstract
In chronic inflammatory pain (CIP) and depression, neuroapoptosis has been identified as a contributing factor. Electroacupuncture (EA) shows promise as an alternative therapy for this comorbidity. However, the underlying mechanism remains unclear. This study aimed to investigate the effects of EA on hippocampal neuronal apoptosis in rats with CIP (chronic inflammatory pain) and depression. Rats received plantar injections of complete Freund’s adjuvant (CFA) on days 0 and 14. They were then divided into groups: sham operation, model, EA, and duloxetine. EA was administered at Hegu (LI4) and Taichong (LR3) from days 15 to 28, while the duloxetine (also known as Cymbalta) group received duloxetine and distilled water daily (0.1 mg/ml). Pain behavior was assessed using the mechanical withdrawal threshold (MWT) and thermal withdrawal latency (TWL) tests. Depression-like behavior was evaluated through the sucrose preference test (SPT), open-field test (OFT), and forced swim test (FST). Hematoxylin and eosin (HE) staining was employed to assess pathological changes in the hippocampus. Nerve cell apoptosis was determined using TUNEL fluorescence staining. Western blot analysis was conducted to measure the protein expression of Bcl-2, Bax, p-PI3K/PI3K, and p-Akt/Akt. EA demonstrated significant pain intensity reduction and alleviation of pain-related depressive symptoms. Our findings from the HE staining confirmed that CIP induced by CFA led to morphological changes in the hippocampus, while EA effectively reversed these pathological alterations. Moreover, EA intervention remarkably reduced neuronal apoptosis and exhibited an upregulation of Bcl-2 protein expression accompanied by a decrease in Bax expression. Additionally, EA activated the PI3K/Akt signaling pathway. Overall, our study suggests that EA holds the potential to improve pain and depressive behaviors in rats with CIP (chronic inflammatory pain) and depression comorbidity, potentially mediated through the activation of the PI3K/Akt pathway, leading to a reduction in hippocampal neuronal apoptosis.
Keywords: Apoptosis; Chronic inflammatory pain; Depression; Electroacupuncture; Neural cells; PI3K/Akt signaling pathway.