Helminth Immunomodulation in Autoimmune Disease.
Kristen Sparrow • June 26, 2022

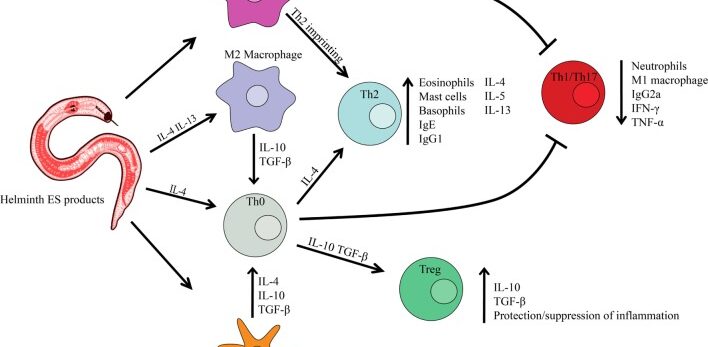
 This study relates to my interest in ways to modulate an overactive immune system. One radical way is to inoculate the patient with hookworm. Because hookworm has learned how to evade the immune system and coexist with humans, the thought is that it can put the brakes on immune overreaction. It doesn’t work reliably, unfortunately, but it still sheds light on different pathways that might be used to calm the auto-immune response.
This study relates to my interest in ways to modulate an overactive immune system. One radical way is to inoculate the patient with hookworm. Because hookworm has learned how to evade the immune system and coexist with humans, the thought is that it can put the brakes on immune overreaction. It doesn’t work reliably, unfortunately, but it still sheds light on different pathways that might be used to calm the auto-immune response.
Smallwood TB, Giacomin PR, Loukas A, Mulvenna JP, Clark RJ, Miles JJ. Helminth Immunomodulation in Autoimmune Disease. Front Immunol. 2017;8:453. Published 2017 Apr 24. doi:10.3389/fimmu.2017.00453
Abstract
Helminths have evolved to become experts at subverting immune surveillance. Through potent and persistent immune tempering, helminths can remain undetected in human tissues for decades. Redirecting the immunomodulating “talents” of helminths to treat inflammatory human diseases is receiving intensive interest. Here, we review therapies using live parasitic worms, worm secretions, and worm-derived synthetic molecules to treat autoimmune disease. We review helminth therapy in both mouse models and clinical trials and discuss what is known on mechanisms of action. We also highlight current progress in characterizing promising new immunomodulatory molecules found in excretory/secretory products of helminths and their potential use as immunotherapies for acute and chronic inflammatory diseases.
Conclusion
With the accruing global burden of autoimmune disease, helminths have become of heightened scientific interest due to their ability to activate immunoregulatory circuits and control immunity. There is strong evidence in mouse models that helminthic therapy, ES components, and helminth-derived synthetic molecules can treat and/or prevent inflammatory diseases such as IBD, T1D, MS, RA, and asthma. Thus far, human trials in celiac disease, UC, CD, MS, RA, and psoriasis have established that therapy is safe with some evidence of therapeutic effect. However, results in the first wave of human trials are not as striking as mouse disease models. Discordance in mouse/human translation is certainly not unique to this system, as is well known in other settings for a number of reasons (188). Of note, a number of the clinical studies conducted to date were not controlled, comprised small sample sizes, and/or did not use human-tropic helminths. Forthcoming trials will directly address these limitations. Going forward, the concurrent development of helminth-derived anti-inflammatory molecules provides many novel opportunities for safer and more controllable therapeutics against chronic inflammatory diseases. Indeed, inclusive efforts in characterizing and mimicking the full immunomodulating abilities of helminths are only in their infancy and much potential exists in this space.

